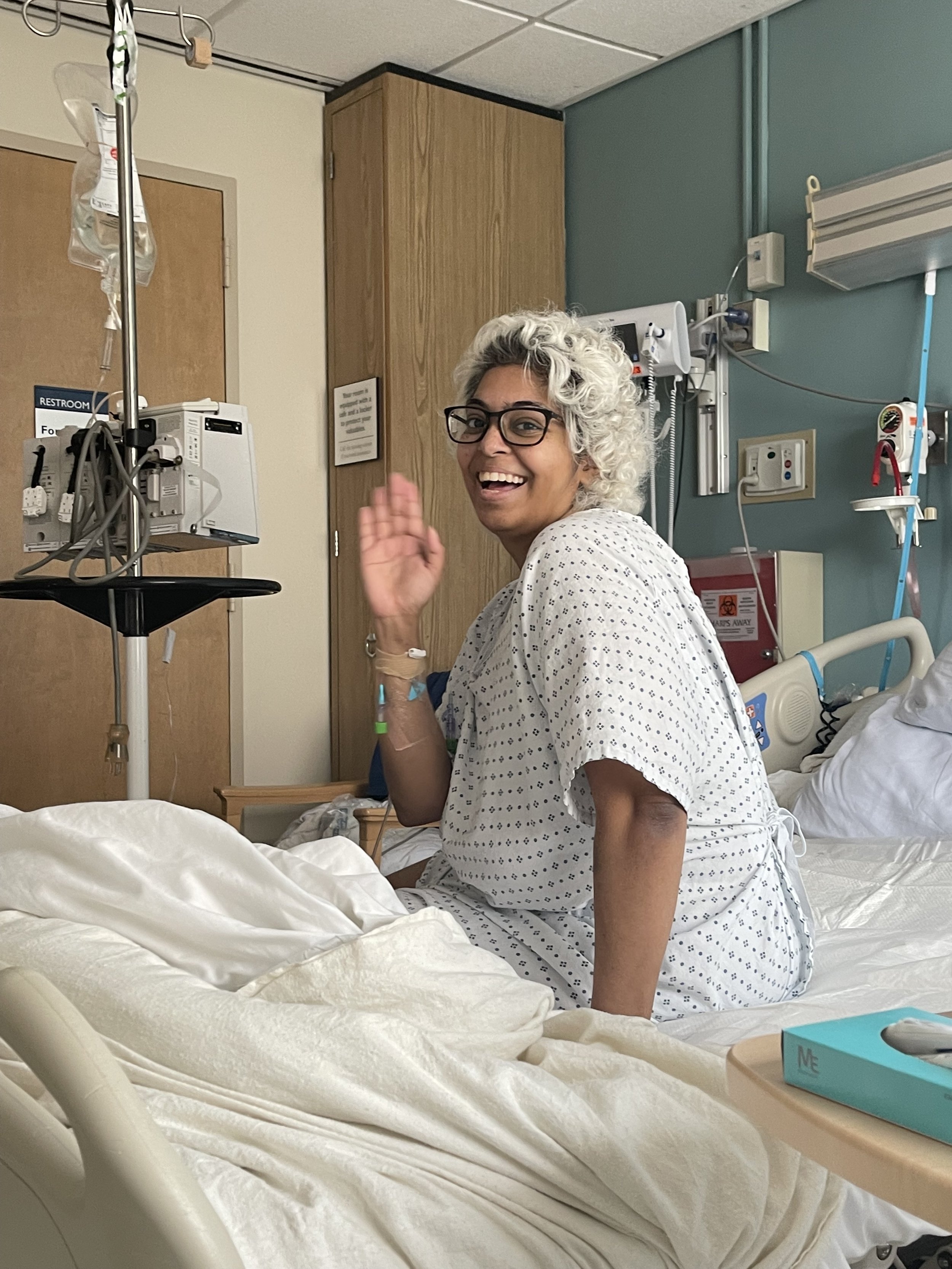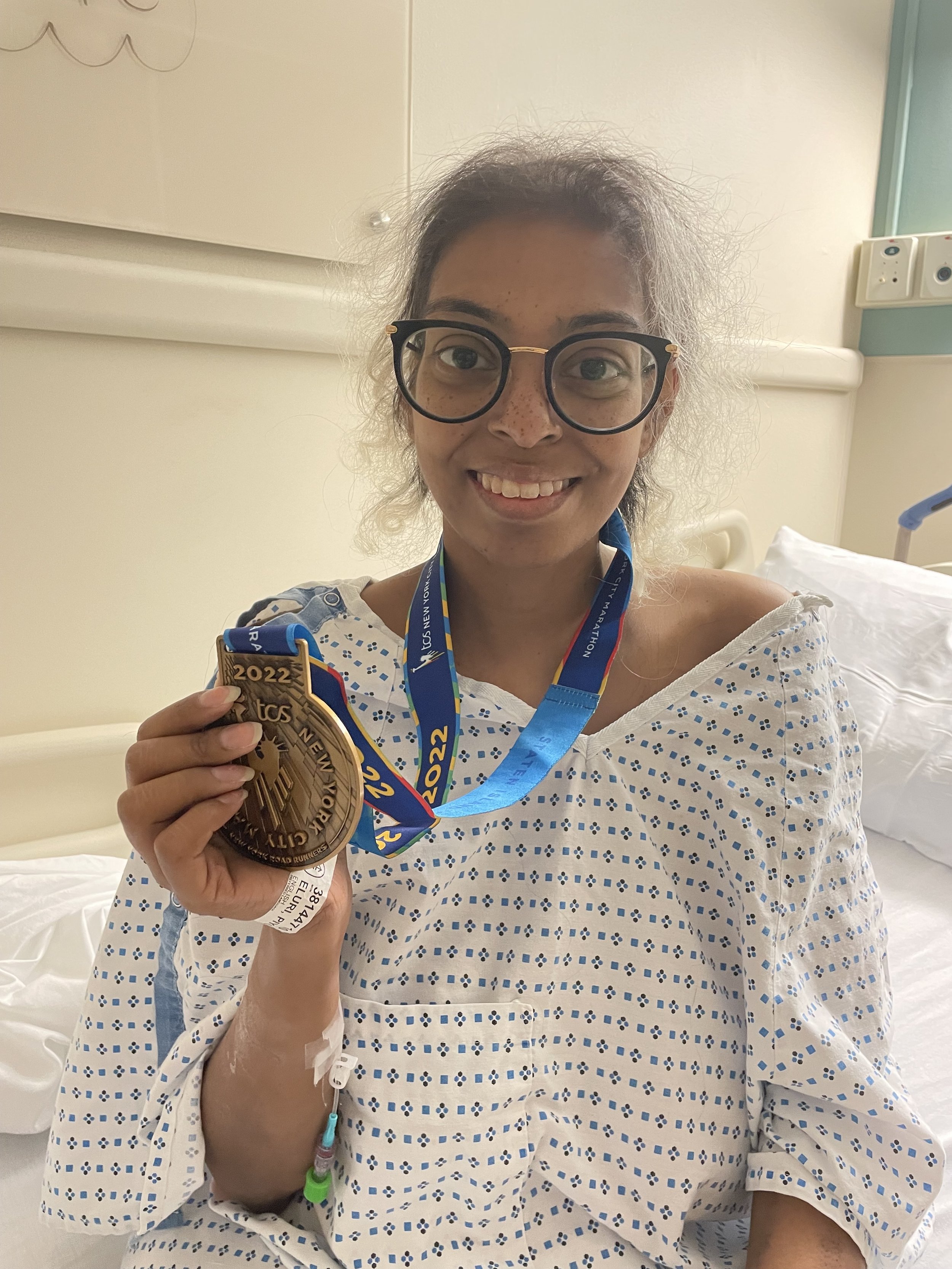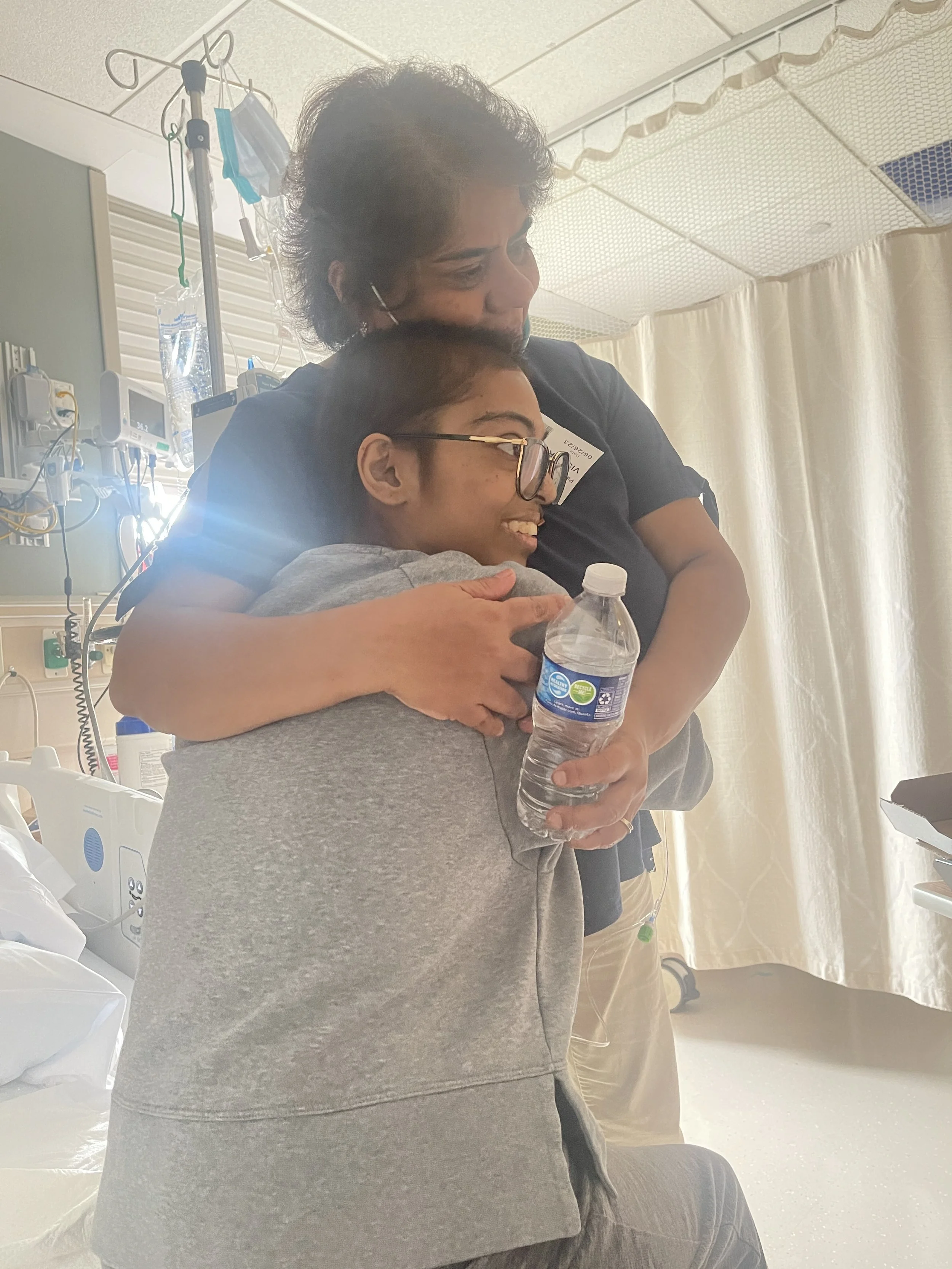
Anaplastic Sarcoma of the Kidney
The 30th recorded case.
Treatment Timeline
Oct. 2020Initial Symptoms
Jan 2021Left Radical Nephrectomy Of 20cm Mass
June 2021AIM Chemo
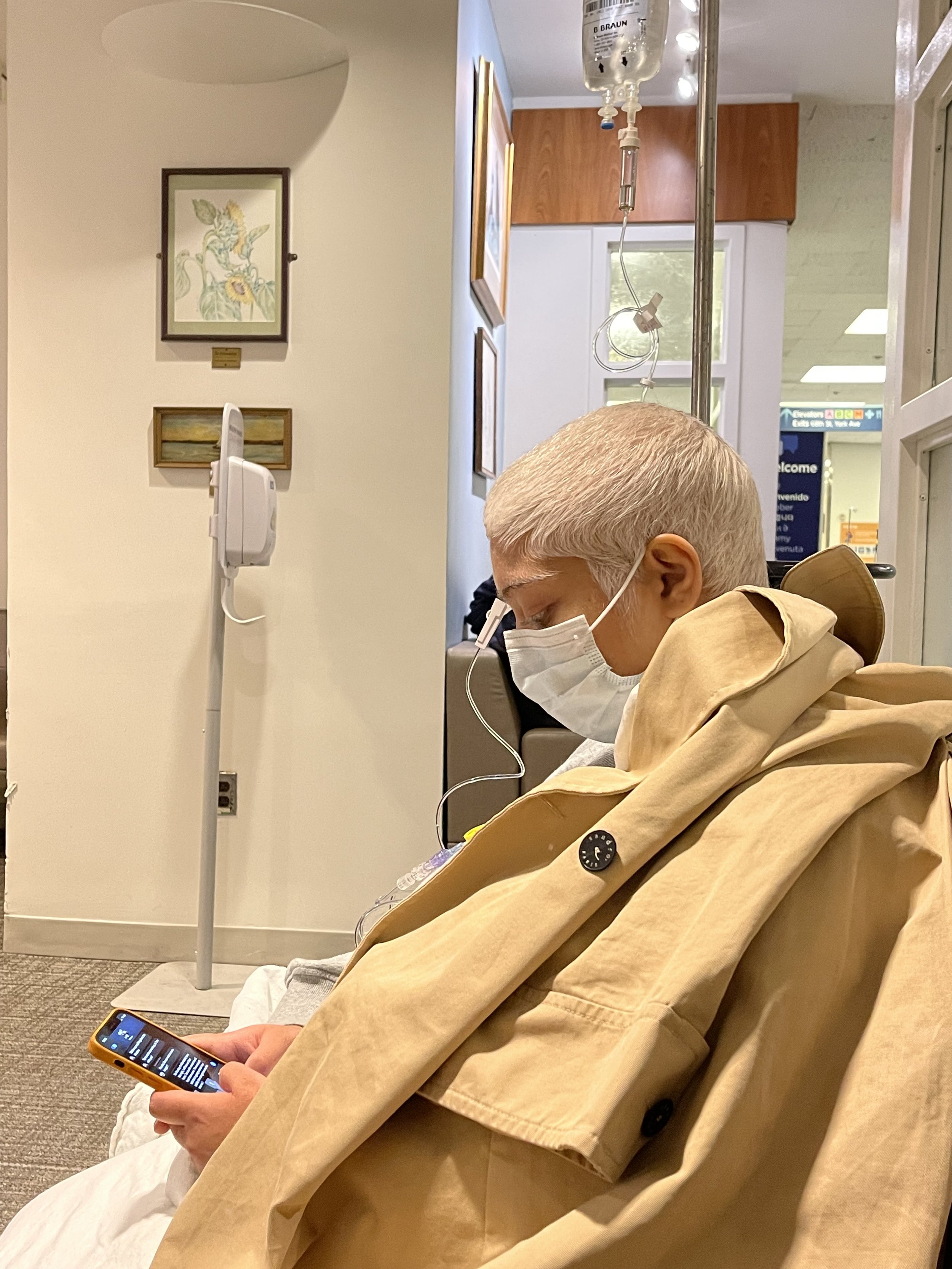
July 2021 - Jan. 2022Avapritinib TKI - n of 1 clinical trial
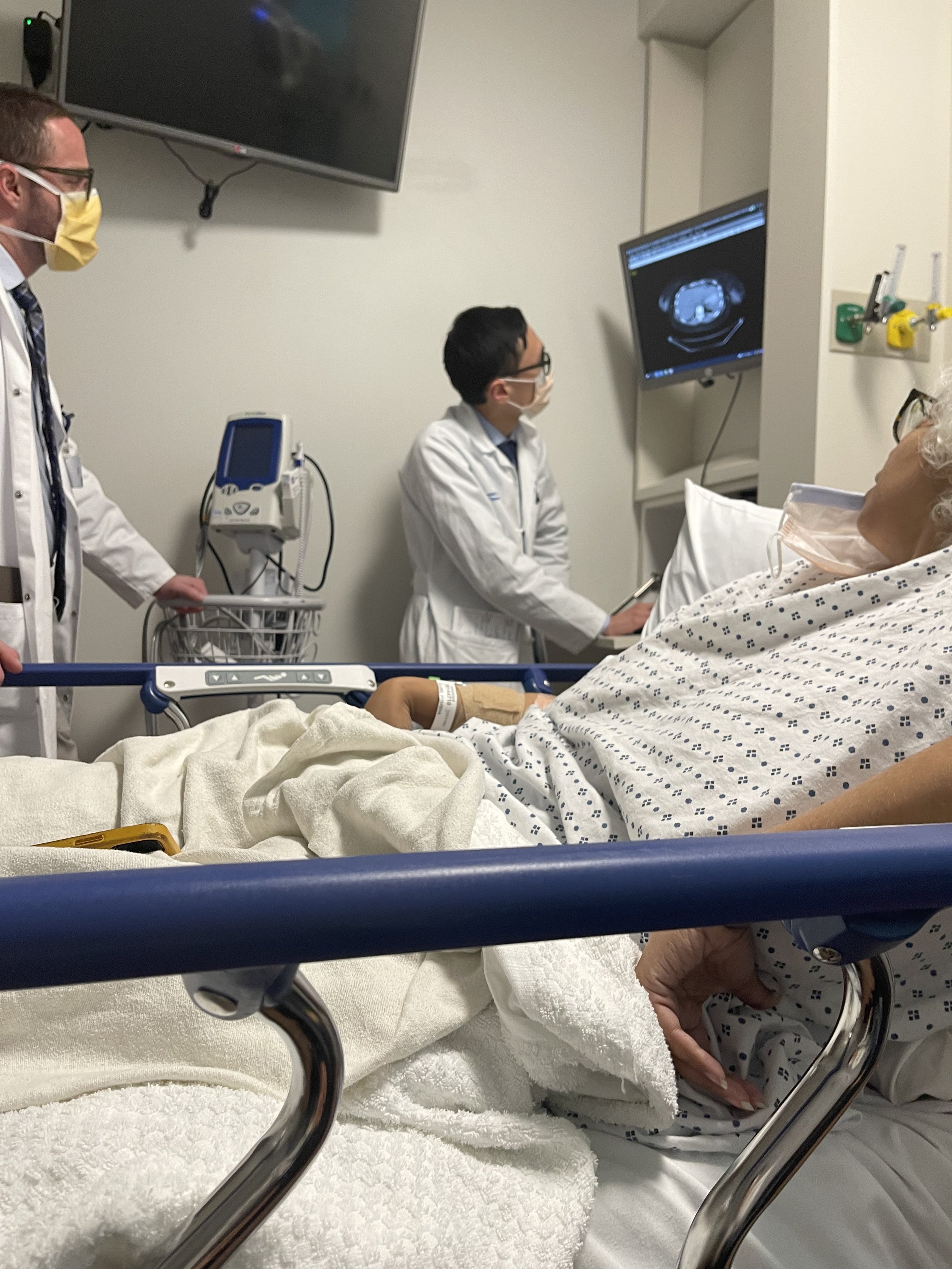
March 2022Debulking Surgery
May 2022Small Bowel Bypass Surgery, PFO
June 2022 - Sept. 2022VIT Chemo
Oct. 2022Rechallenge Avapritinib, IE Chemo
Nov. 2022Embolic Stroke, Neutropenic, Collapsed Lung, Fungemia
Jan. - May 2023CT Chemo
May 2023GV Chemo
June 2023TI Chemo
Initial Symptoms
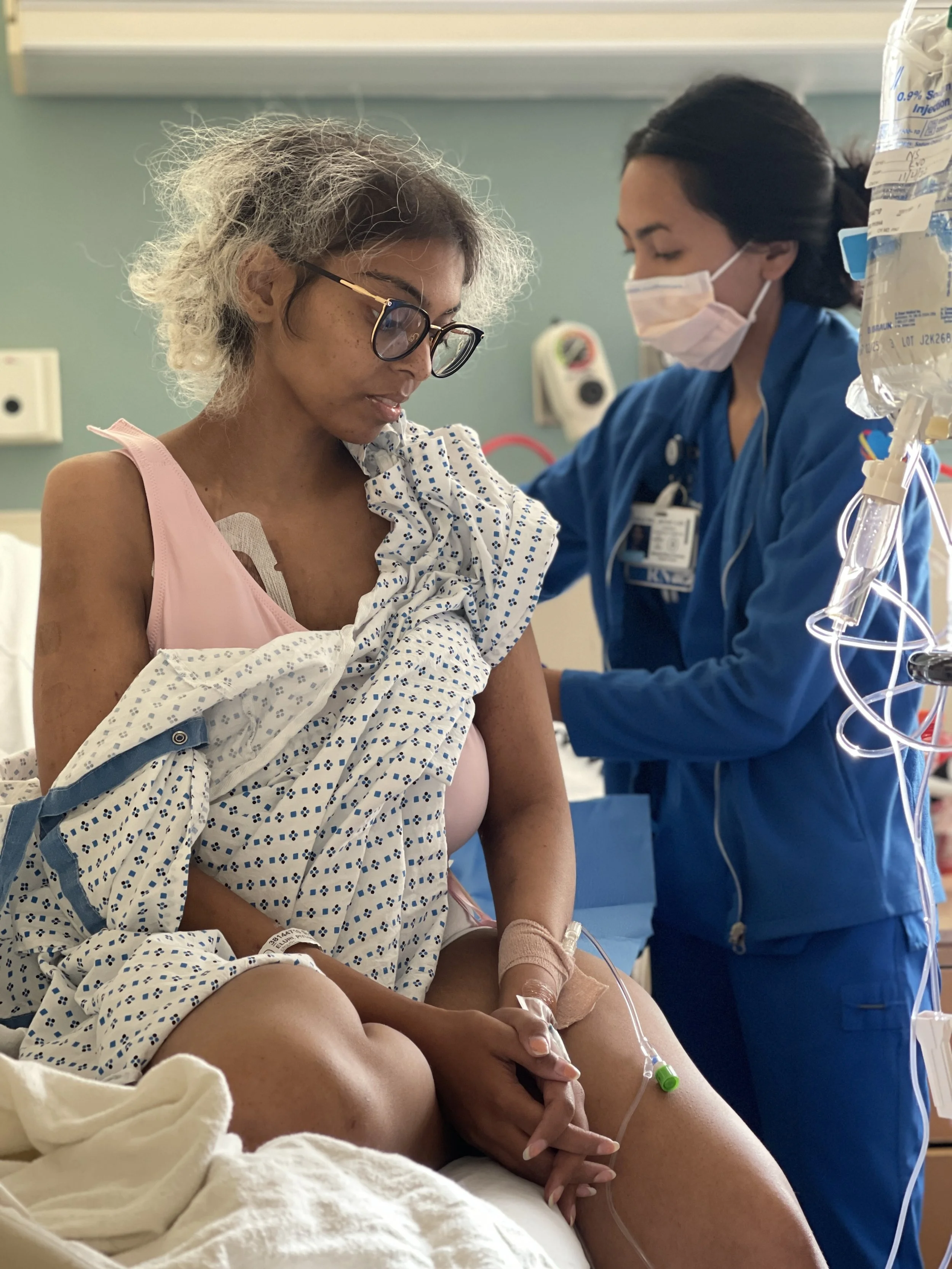
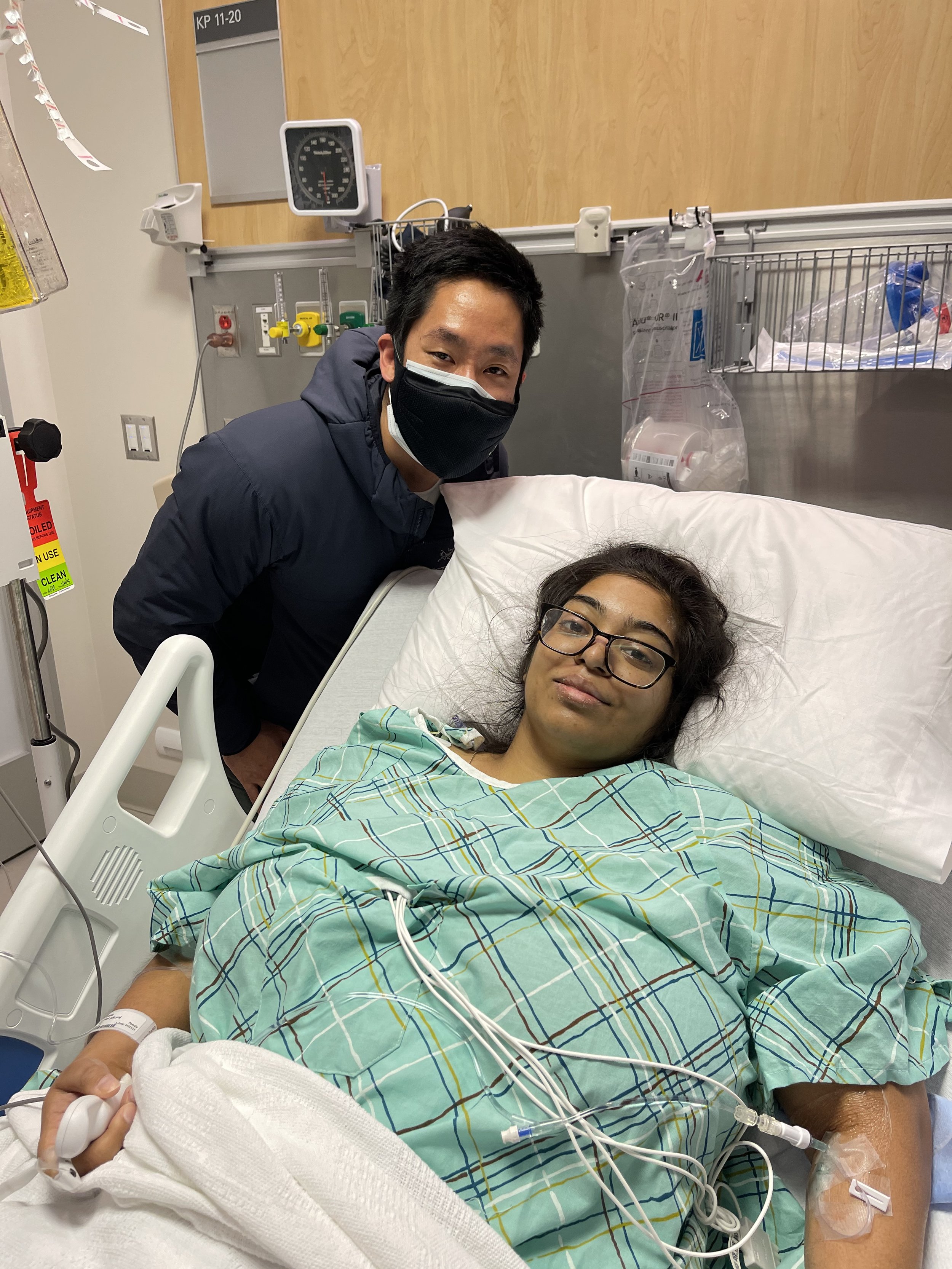
2 weeks after our civil marriage was when her first symptoms of back pain had set in during a Hudson Valley trip with friends. She spent a month escalating it with her PCP and OBGYN before her Gastroenterologist decided to order a CT scan and that was when we found an 8cm mass and rushed to the ER. Still peak covid, moments after the ER resident told us she might have cancer, I was asked to leave the hospital.
Nephrectomy
After 1.5 months of at home care and an uncertain diagnosis, Piyusha was rushed into emergency surgery on January 22, 2021 which was successful with margins.
Recurrence
After 5 months of her clinical profile and cell block being sent to pathologists around the nation, we were referred to Memorial Sloan Kettering for care due to the uncertainty of her diagnosis. With the help of modern gene testing from Dr. Ashley Hill at Children’s National and Memorial Sloan Kettering’s IMPACT (part funded by Cycle For Survival), we were able to identify 2 mutations within her tumors (PDGFRAD842V & DICER1).
The Whitepaper
Given the presence of two rare gene mutations (PDGFRAD842V & DICER1), there was no playbook for treatment for a patient like Piyusha. Avapritinib, a newly developed tyrosine kinase inhibitor showed promise for one of her mutations but was not approved for her use due to her clinical profile. We were lucky to have the support of our research oncologist Dr. Ping Chi at Memorial Sloan Kettering, who was able to get compassionate use authorization from the manufacturer in exchange for an n of 1 clinical trial.
“Avapritinib was just approved by the FDA last December (right in time!) and I am a one-person clinical trial, as it has never been used on this tumor type. We are feeling optimistic, grateful for access to the medication and MSK, and also thankful that the side effects of this treatment are slightly less intensive that chemo. I officially started this treatment today!”
-Piyusha on July 23, 2021
Work In Progress